Naproxen
Chemical Name | :Naproxen |
Category | :Antipyretic-analgesic and Anti-inflammatory |
Specification | :USP/EP |
HS Code | :29189900.90 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :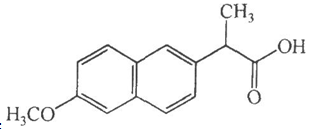 |
CAS Number | :22204-53-1 |
Molecular Formula | :C14H14O3 230.26 |
Usage | :The product is PG synthetase inhibitor having anti-inflammatory, antipyretic and analgesic effect. It has positive effect on Rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, gout and motor system (such as joints, muscles and tendons) of chronic degenerative diseases and mild and moderate pain such as dysmenorrhea. Medium-degree pain can be relieved in 1 hour after taking the drug, whose abirritation can up to 7 hours. Its curative effect is similar to aspirin on the treatment of rheumatoid arthritis and osteoarthritis. For patient who can’t tolerance anti-inflammatory analgesic (such as aspirin, indomethacin) because of Anemia, gastrointestinal system disease or other reasons, using this product can usually get satiafactory effect. |
Items | Standard |
|---|---|
Appearance | White or almost white, crystalline powder |
Solubility | Practically insoluble in water, soluble in rthanol(96%) and in methanol |
Identification Specific optical rotation Melting point Infrared absorption | +59°~+62° 154℃~158℃ Positive |
Appearance of solution | Clear, not more intensively colored than reference solution BY7 |
Enantiomeric Impurities Impurity G | ≤2.5% |
Ralated substances Impurity L Impurity O Any other impurity Total impurities | ≤0.15% ≤0.15% ≤0.10% ≤0.3% |
Heavy Metals | ≤20ppm |
Loss on drying | ≤0.5% |
Sulphated ash | ≤0.1% |
Assay (titration, on dried substance) | 99.0%~101.0% |
Residual solvent Methanol Toluene | ≤50ppm ≤45ppm |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian?Therapeutic?Goods?Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian?Health?Products and?Food?Branch?Inspectorate(HPFBI)
5, Taiwan?Food and?Drug?Administration?(TFDA)
7, Czech State Institute for Drug Control
Státní ústav pro Kontrolu Lé?iv (SúKL)
8, Czech?Institute for?State?Control of?Veterinary?Biologicals?and?Medicines (ISCVBM)
9, Danish?Health and?Medicines?Authority (DHMA)
10, Finnish?Medicines?Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, 13, German Federal Ministry of Health * 14, Icelandic?Medicines?Agency?(IMA) 20, Indonesian?National?Agency for?Drug and?Food?Control (NADFC) 21, Health?Products?Regulatory?Authority?(HPRA) 22, Italian Medicines Agency 23, Japanese?Pharmaceuticals and?Medical?Devices?Agency(PMDA) 24, Korea (Republic of)?Ministry of?Food and?Drug?Safety?(MFDS) 25, Malaysian?National?Pharmaceutical?Control?Bureau?(NPCB) 26, Dutch Health Care Inspectorate* 27, (Medsafe) 28, Norwegian?Medicines?Agency?(NOMA) 29, Polish?Main?Pharmaceutical?Inspectorate (MPI) 30, Romanian?National?Agency for?Medicines and?MedicalDevices (NAMMD) 31, South African?Medicines?Control?Council?(MCC) 32, Spanish Agency of Medicines and Medical Devices?* Agencia Espa?ola de Medicamentos y Productos Sanitarios(AEMPS) 33, Swedish?Medical?Products?Agency?(MPA) 34, (MHRA) 36, U.S.?Food and?Drug?Administration (US FDA) 37, European Directorate for the Quality of Medicines & HealthCare (EDQM) 38, European Medicines Agency(EMA) 39, World Health Organization(WHO)
Bundesministerium für Gesundheit (BMG)
Agenzia Italiana del Farmaco (AIFA)
Inspectie voor de Gezondheidszorg (IGZ)