Veterinary / PRODUCT
HOME>Product>Animal Health>Veterinary
Levamisole Hcl
Chemical Name | :Levamisole Hcl |
Category | :Anthelmintic |
Specification | :BP/EP |
HS Code | :29349990.22 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :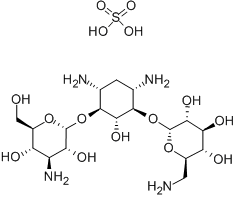 |
CAS Number | :133-92-6 |
Molecular Formula | :C18H38N4O15S |
Usage | :Have immune adjustment and immune stimulation.The roundworm, hookworm, pinworm and dung round nematode disease has good curative effect. |
Items | Standard |
|---|---|
Appearance | A white or almost white crystalline power |
Solubility | Freely soluble in water,soluble in ethanol ,slightly soluble in methylene chloride. |
Specific Optical Rotation | -121° to -128° |
Infrared absorption | Conform to the spectrum obtained from Levamisole Hydrochloride RS |
Specific optical rotation | Complise |
Chemical reaction | Positive |
pH | 3.0~4.5 |
Related substance | Impurities A,B,C,D,E: For each impurity≤ 0.2%. Other individual unspecified impurities≤0.10% Total of all impurities≤0.3% |
Heavy metals | ≤20ppm |
Loss on drying | ≤0.5% |
Sulfated ash | ≤0.1% |
Assay (calculated on the dried basis) | 98.5% ~ 101.0% |
Appearance of solution | Complies |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian?Therapeutic?Goods?Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian?Health?Products and?Food?Branch?Inspectorate(HPFBI)
5, Taiwan?Food and?Drug?Administration?(TFDA)
7, Czech State Institute for Drug Control
Státní ústav pro Kontrolu Lé?iv (SúKL)
8, Czech?Institute for?State?Control of?Veterinary?Biologicals?and?Medicines (ISCVBM)
9, Danish?Health and?Medicines?Authority (DHMA)
10, Finnish?Medicines?Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, 13, German Federal Ministry of Health * 14, Icelandic?Medicines?Agency?(IMA) 20, Indonesian?National?Agency for?Drug and?Food?Control (NADFC) 21, Health?Products?Regulatory?Authority?(HPRA) 22, Italian Medicines Agency 23, Japanese?Pharmaceuticals and?Medical?Devices?Agency(PMDA) 24, Korea (Republic of)?Ministry of?Food and?Drug?Safety?(MFDS) 25, Malaysian?National?Pharmaceutical?Control?Bureau?(NPCB) 26, Dutch Health Care Inspectorate* 27, (Medsafe) 28, Norwegian?Medicines?Agency?(NOMA) 29, Polish?Main?Pharmaceutical?Inspectorate (MPI) 30, Romanian?National?Agency for?Medicines and?MedicalDevices (NAMMD) 31, South African?Medicines?Control?Council?(MCC) 32, Spanish Agency of Medicines and Medical Devices?* Agencia Espa?ola de Medicamentos y Productos Sanitarios(AEMPS) 33, Swedish?Medical?Products?Agency?(MPA) 34, (MHRA) 36, U.S.?Food and?Drug?Administration (US FDA) 37, European Directorate for the Quality of Medicines & HealthCare (EDQM) 38, European Medicines Agency(EMA) 39, World Health Organization(WHO)
Bundesministerium für Gesundheit (BMG)
Agenzia Italiana del Farmaco (AIFA)
Inspectie voor de Gezondheidszorg (IGZ)