API / PRODUCT
HOME>Product>Human Health>API
Fenofibrate
Chemical Name | :Fenofibrate |
Category | : |
Specification | :EP/BP/USP |
HS Code | :29189900.90 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :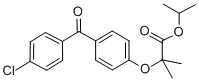 |
CAS Number | :49562-28-9 |
Molecular Formula | :C20H21ClO4 |
Usage | :This product has a significant function of lowering serum cholesterol under the effect of esterase rapidly metabolized in the body of fenofibrate acid and landing blood lipid and lowering triglyceride level and increase high density lipoprotein cholesterol. It is suitable for the treatment of high blood triglycerides and hypercholesterolemia, whose curative effect is superior to the chlorine Bert with less side effects. |
Items | Standard |
|---|---|
Appearance | White or slightly yellow, crystalline powder |
Solubility | Practically insoluble in water, freely soluble in anhydrous ethanol, soluble in toluene. |
Melting point | 116 – 118℃ |
Identification | IR: Should be identical to that of RS |
Appearance of solution | Clear, not more than BY4 |
Water | ≤0.5% |
Chlorides | ≤350ppm |
Sulphated ash | ≤0.1% |
Related substances | Impurity A≤0.1% Impurity B≤0.2% Impurity C≤0.1% Impurity D≤0.1% Impurity E≤0.8% Any other impurity ≤0.1% Total of other impurities ≤0.5% |
Assay | 99%-101%(anhydrous substance) |
Residual solvents | Toluene≤890ppm Hexane≤290ppm |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian?Therapeutic?Goods?Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian?Health?Products and?Food?Branch?Inspectorate(HPFBI)
5, Taiwan?Food and?Drug?Administration?(TFDA)
7, Czech State Institute for Drug Control
Státní ústav pro Kontrolu Lé?iv (SúKL)
8, Czech?Institute for?State?Control of?Veterinary?Biologicals?and?Medicines (ISCVBM)
9, Danish?Health and?Medicines?Authority (DHMA)
10, Finnish?Medicines?Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, 13, German Federal Ministry of Health * 14, Icelandic?Medicines?Agency?(IMA) 20, Indonesian?National?Agency for?Drug and?Food?Control (NADFC) 21, Health?Products?Regulatory?Authority?(HPRA) 22, Italian Medicines Agency 23, Japanese?Pharmaceuticals and?Medical?Devices?Agency(PMDA) 24, Korea (Republic of)?Ministry of?Food and?Drug?Safety?(MFDS) 25, Malaysian?National?Pharmaceutical?Control?Bureau?(NPCB) 26, Dutch Health Care Inspectorate* 27, (Medsafe) 28, Norwegian?Medicines?Agency?(NOMA) 29, Polish?Main?Pharmaceutical?Inspectorate (MPI) 30, Romanian?National?Agency for?Medicines and?MedicalDevices (NAMMD) 31, South African?Medicines?Control?Council?(MCC) 32, Spanish Agency of Medicines and Medical Devices?* Agencia Espa?ola de Medicamentos y Productos Sanitarios(AEMPS) 33, Swedish?Medical?Products?Agency?(MPA) 34, (MHRA) 36, U.S.?Food and?Drug?Administration (US FDA) 37, European Directorate for the Quality of Medicines & HealthCare (EDQM) 38, European Medicines Agency(EMA) 39, World Health Organization(WHO)
Bundesministerium für Gesundheit (BMG)
Agenzia Italiana del Farmaco (AIFA)
Inspectie voor de Gezondheidszorg (IGZ)