Ciprofloxacin
Chemical Name | :Ciprofloxacin |
Category | :The third generation ofquinolone antibiotics |
Specification | :EP/USP |
HS Code | :30049090.90 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :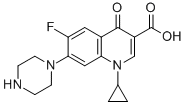 |
CAS Number | :85721-33-1 |
Molecular Formula | :C17H18FN3O3 |
Usage | :Ciprofloxacin is the synthesis of the third generation quinolone antibacterial drugs, with broad-spectrum antibacterial activity and good bactericidal effect. Its effect of antibacterial activity is 2~4 times stronger than that of norfloxacin and enoxacin for almost all bacteria.It has antibacterial effect on e. coli, pseudomonas aeruginosa, h. influenzae, neisseria gonorrhoeae, legionella bacteria, staphylococcus aureus, streptococcus. |
Items | Standard |
|---|---|
Appearance | White to off-white, ordorless,crystalline powder |
Identification | A: IR B: UV |
Specific optical rotation | +45~+51° |
Chromatographic purity Any individual impurity Total impurities | ≤1.0% ≤1.5% |
Ralated compound A | ≤0.5% |
Melting point | About 205℃ |
Loss on drying | ≤1.0% |
Solubility | Freely soluble in choroform; sparingly soluble in alcohol and in methanol;slightly soluble in ether; insoluble in water. |
Residual solvents Methanol Methylene chloride | ≤3000ppm ≤500ppm |
Assay | 97.0~103.0% |
Micron | 100%≤20μm |
Conclusion | Conform the stipulation |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian?Therapeutic?Goods?Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian?Health?Products and?Food?Branch?Inspectorate(HPFBI)
5, Taiwan?Food and?Drug?Administration?(TFDA)
7, Czech State Institute for Drug Control
Státní ústav pro Kontrolu Lé?iv (SúKL)
8, Czech?Institute for?State?Control of?Veterinary?Biologicals?and?Medicines (ISCVBM)
9, Danish?Health and?Medicines?Authority (DHMA)
10, Finnish?Medicines?Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, 13, German Federal Ministry of Health * 14, Icelandic?Medicines?Agency?(IMA) 20, Indonesian?National?Agency for?Drug and?Food?Control (NADFC) 21, Health?Products?Regulatory?Authority?(HPRA) 22, Italian Medicines Agency 23, Japanese?Pharmaceuticals and?Medical?Devices?Agency(PMDA) 24, Korea (Republic of)?Ministry of?Food and?Drug?Safety?(MFDS) 25, Malaysian?National?Pharmaceutical?Control?Bureau?(NPCB) 26, Dutch Health Care Inspectorate* 27, (Medsafe) 28, Norwegian?Medicines?Agency?(NOMA) 29, Polish?Main?Pharmaceutical?Inspectorate (MPI) 30, Romanian?National?Agency for?Medicines and?MedicalDevices (NAMMD) 31, South African?Medicines?Control?Council?(MCC) 32, Spanish Agency of Medicines and Medical Devices?* Agencia Espa?ola de Medicamentos y Productos Sanitarios(AEMPS) 33, Swedish?Medical?Products?Agency?(MPA) 34, (MHRA) 36, U.S.?Food and?Drug?Administration (US FDA) 37, European Directorate for the Quality of Medicines & HealthCare (EDQM) 38, European Medicines Agency(EMA) 39, World Health Organization(WHO)
Bundesministerium für Gesundheit (BMG)
Agenzia Italiana del Farmaco (AIFA)
Inspectie voor de Gezondheidszorg (IGZ)