Azithromycin
Chemical Name | :Azithromycin |
Category | :Macrolides Antibiotics |
Specification | :USP/EP |
HS Code | :2941.9090.00 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :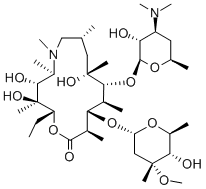 |
CAS Number | :83905-01-5 |
Molecular Formula | :C38H72N2O12 |
Usage | :Macrolide antibiotics, 15 yuan of nitrogen containing heterocyclic, antibacterial mechanism and erythromycin is similar, but the broad antimicrobial spectrum. The gram positive bacterium stronger antibacterial activity, also has strong antibacterial activity against gram negative bacteria such as Haemophilus influenzae, Escherichia coli, Salmonella, Shigella and etc.. And the acid stability, good tolerance. Are due to the sensitive strains of respiratory tract infections, skin and soft tissue infections, sexually transmitted diseases and other good therapeutic effect. |
Items | Standard |
|---|---|
Appearance | A white or almost white, crystalline powder. Practically insoluble in water, freely soluble in anhydrous ethanol and in methylene chloride. |
Identification | (1) IR: Conforms to the Azithromycin RS spectrum (2) HPLC: The retention time of azithromycin peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay. |
Specific rotation | -45°~-49°(Anhydrous substance 20mg/ml dehydrated alcohol) |
Crystallinity | Meets the requirements |
PH | 9.0~11.0 ( 0.1g /50ml methanol-water (1:1)) |
Water | 4.0%~5.0% |
Residue on Ignition | ≤0.3% |
Heavy metals | ≤0.0025% |
Residual solvents | (1) Methylene Chloride (2) Acetone |
Assay (HPLC) | C38H72N2O12 945~1030 μg /mg 945~1030 μg /mg |
We support the below registration services:
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian?Therapeutic?Goods?Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian?Health?Products and?Food?Branch?Inspectorate(HPFBI)
5, Taiwan?Food and?Drug?Administration?(TFDA)
7, Czech State Institute for Drug Control
Státní ústav pro Kontrolu Lé?iv (SúKL)
8, Czech?Institute for?State?Control of?Veterinary?Biologicals?and?Medicines (ISCVBM)
9, Danish?Health and?Medicines?Authority (DHMA)
10, Finnish?Medicines?Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, 13, German Federal Ministry of Health * 14, Icelandic?Medicines?Agency?(IMA) 20, Indonesian?National?Agency for?Drug and?Food?Control (NADFC) 21, Health?Products?Regulatory?Authority?(HPRA) 22, Italian Medicines Agency 23, Japanese?Pharmaceuticals and?Medical?Devices?Agency(PMDA) 24, Korea (Republic of)?Ministry of?Food and?Drug?Safety?(MFDS) 25, Malaysian?National?Pharmaceutical?Control?Bureau?(NPCB) 26, Dutch Health Care Inspectorate* 27, (Medsafe) 28, Norwegian?Medicines?Agency?(NOMA) 29, Polish?Main?Pharmaceutical?Inspectorate (MPI) 30, Romanian?National?Agency for?Medicines and?MedicalDevices (NAMMD) 31, South African?Medicines?Control?Council?(MCC) 32, Spanish Agency of Medicines and Medical Devices?* Agencia Espa?ola de Medicamentos y Productos Sanitarios(AEMPS) 33, Swedish?Medical?Products?Agency?(MPA) 34, (MHRA) 36, U.S.?Food and?Drug?Administration (US FDA) 37, European Directorate for the Quality of Medicines & HealthCare (EDQM) 38, European Medicines Agency(EMA) 39, World Health Organization(WHO)
Bundesministerium für Gesundheit (BMG)
Agenzia Italiana del Farmaco (AIFA)
Inspectie voor de Gezondheidszorg (IGZ)