Amikacin Sulfate
Chemical Name | :Amikacin Sulfate |
Category | :Antibiotics |
Specification | :EP/USP |
HS Code | :29419020.00 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :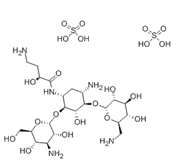 |
CAS Number | :149022-22-0 |
Molecular Formula | :C22H43N5O13·nH2SO4(n=1.8) |
Usage | :It is suitable for livestock and poultry digestive tract infection, peritonitis, salpingitis pullorosis, avian typhoid, paratyphoid, poultry chicken escherichia coli disease, singular proteus disease, pseudomonas aeruginosa, staphylococcus aureus, pullorosis artificially infected in yellow, piglets, pigs pant disease, infectious gastroenteritis, swine erysipelas, swine atrophic rhinitis, necrotic enteritis and urinary tract diseases. Aquatic products: bacterial septicemia, enteritis, red skin disease, ascites, disease, etc. |
Items | Standard |
|---|---|
Characters | White or almost white crystalline powder |
Identification TCL
HPLC | A. The spots obtained from the test solution and mmised solution are similar in position and color to that obtained with reference solution. B. The retention time of the peak in the chromatogram of the Assay corresponds to that in the chromatogram of the reference solution. |
Specific rotation | +76°~+84° |
Crystallinity | Meet the requirements |
PH | 2.0-4.0 |
Loss on dry | ≤13.0 % |
Residue on ignition | ≤1.0% |
Residual solvents -Methanol -Ethanol -Acetone -Acetonitrile | ≤0.3% ≤0.5% ≤0.5% ≤0.041% |
Assay(dried substance) | 674-786ug/mg |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian?Therapeutic?Goods?Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian?Health?Products and?Food?Branch?Inspectorate(HPFBI)
5, Taiwan?Food and?Drug?Administration?(TFDA)
7, Czech State Institute for Drug Control
Státní ústav pro Kontrolu Lé?iv (SúKL)
8, Czech?Institute for?State?Control of?Veterinary?Biologicals?and?Medicines (ISCVBM)
9, Danish?Health and?Medicines?Authority (DHMA)
10, Finnish?Medicines?Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, 13, German Federal Ministry of Health * 14, Icelandic?Medicines?Agency?(IMA) 20, Indonesian?National?Agency for?Drug and?Food?Control (NADFC) 21, Health?Products?Regulatory?Authority?(HPRA) 22, Italian Medicines Agency 23, Japanese?Pharmaceuticals and?Medical?Devices?Agency(PMDA) 24, Korea (Republic of)?Ministry of?Food and?Drug?Safety?(MFDS) 25, Malaysian?National?Pharmaceutical?Control?Bureau?(NPCB) 26, Dutch Health Care Inspectorate* 27, (Medsafe) 28, Norwegian?Medicines?Agency?(NOMA) 29, Polish?Main?Pharmaceutical?Inspectorate (MPI) 30, Romanian?National?Agency for?Medicines and?MedicalDevices (NAMMD) 31, South African?Medicines?Control?Council?(MCC) 32, Spanish Agency of Medicines and Medical Devices?* Agencia Espa?ola de Medicamentos y Productos Sanitarios(AEMPS) 33, Swedish?Medical?Products?Agency?(MPA) 34, (MHRA) 36, U.S.?Food and?Drug?Administration (US FDA) 37, European Directorate for the Quality of Medicines & HealthCare (EDQM) 38, European Medicines Agency(EMA) 39, World Health Organization(WHO)
Bundesministerium für Gesundheit (BMG)
Agenzia Italiana del Farmaco (AIFA)
Inspectie voor de Gezondheidszorg (IGZ)