Cefixime Micronized
Chemical Name | :Cefixime |
Cefixime | :Third-generation oral cephalosporins |
Specification | :USP |
HS Code | :2941.9059.90 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :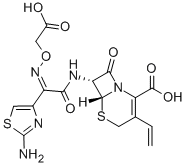 |
CAS Number | :79350-37-1 |
Molecular | :C16H15N5O7S2 |
Usage | :Cefixime antibacterial mechanism similar to other third-generation cephalosporins, by one or more of penicillin binding proteins (PBPs) combined, inhibition of bacterial cell wall synthesis in dividing cells. |
Items | Standard |
|---|---|
Appearance | White or slightly yellow, hygroscopic powder |
Identification | The IR spectrum confirm to that of reference standard |
PH | 2.6 to 4.1 |
Any other impurity | NMT 0.5% |
Total | NMT 0.3% |
Methanol | Not more than 0.3% |
Ethanol | Not more than 0.5% |
Acetone | Not more than 0.5% |
Acetic ether | Not more than 0.5% |
Butylene oxide | Not more than 0.5% |
Water | 9.0% to 12.0% |
Sulphated ash | Not more than 0.2% |
Assay (Sum) | 96.0% to 102.0% |
We support the below registration services:
1, Argentinian National Institute of Drugs Instituto Nacional de Medicamentos (INAME)
2, Australian?Therapeutic?Goods?Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian?Health?Products and?Food?Branch?Inspectorate(HPFBI)
5, Taiwan?Food and?Drug?Administration?(TFDA)
7, Czech State Institute for Drug Control Státní ústav pro Kontrolu Lé?iv (SúKL)
8, Czech?Institute for?State?Control of?Veterinary?Biologicals?and?Medicines (ISCVBM)
9, Danish?Health and?Medicines?Authority (DHMA)
10, Finnish?Medicines?Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, 13, German Federal Ministry of Health * Bundesministerium für Gesundheit (BMG) 14, Icelandic?Medicines?Agency?(IMA) 20, Indonesian?National?Agency for?Drug and?Food?Control (NADFC) 21, Health?Products?Regulatory?Authority?(HPRA) 22, Italian Medicines Agency Agenzia Italiana del Farmaco (AIFA) 23, Japanese?Pharmaceuticals and?Medical?Devices?Agency(PMDA) 24, Korea (Republic of)?Ministry of?Food and?Drug?Safety?(MFDS) 25, Malaysian?National?Pharmaceutical?Control?Bureau?(NPCB) 26, Dutch Health Care Inspectorate* 27, (Medsafe) 28, Norwegian?Medicines?Agency?(NOMA) 29, Polish?Main?Pharmaceutical?Inspectorate (MPI) 30, Romanian?National?Agency for?Medicines and?MedicalDevices (NAMMD) 31, South African?Medicines?Control?Council?(MCC) 32, Spanish Agency of Medicines and Medical Devices?* Agencia Espa?ola de Medicamentos y Productos Sanitarios(AEMPS) 33, Swedish?Medical?Products?Agency?(MPA) 34, (MHRA) 36, U.S.?Food and?Drug?Administration (US FDA) 37, European Directorate for the Quality of Medicines & HealthCare (EDQM) 38, European Medicines Agency(EMA) 39, World Health Organization(WHO)
Inspectie voor de Gezondheidszorg (IGZ)